Presentation
Diagnosis
Research
Covid19
Documentation
Presentation
Diagnosis
Research
-
Detection Kits
- Strept Biotine HRP
- Strept Biotine AP
-
Polymers AP
- Human Tissues Rabbit and Mouse Antibody
- Human and mouse tissues rabbit antibody
- Human Tissues Mouse Antibody
- Human tissues human antibody
- Human tissues mouse rabbit and goat antibody
- Mouse rat and other tissues mouse antibody
- Rat and human tissues mouse antibody
- Human Tissues Goat Antibody
- Human tissues rat antibody
- Mouse and rat tissues human antibody
-
Polymers HRP
- Human Tissues Mouse and Rabbit Antibody
- Human and Rat Tissues Mouse Antibody
- Mouse and Rat Tissues Human Antibody
- Human Tissues Mouse Antibody
- Human Tissues Human Antibody
- Human and Mouse Tissues Rabbit Antibody
- Human Tissues Mouse Rabbit and Goat Antibody
- Human Tissues Goat Antibody
- Human Tissues Rat Antibody
- Mouse Rat and others Tissues Mouse Antibody
- Human Tissues Sheep Antibody
- Human Tissues Guinea Pig Antibody
- Human Tissues Armenian Hamster Antibody
- Human Tissues Chicken Antibody
- Human Tissues Syrian Hamster Antibody
-
Polymers double marquage
- Tissus Human Ac de Lapin et Souris
- Human Tissues 2 Mouse Antibody
- Human Tissues 2 Rabbit Antibody
- Human Tissues Rabbit and Goat Antibody
- Human Tissues Goat and Rat Antibody
- Human Tissues Goat and Mouse Antibody
- Human Tissues Mouse and Rat Antibody
- Mouse Tissues Rat and Mouse Antibody
- Human Tissues Rabbit and Rat Antibody
- Mouse and Rat Tissues 2 Mouse Antibody
- Mouse Tissues Rabbit and Mouse antibody
- Polymers triple marquage
- Strept Réactifs
- Tissues Control
- Chromogènes
- Dyes
- Buffers
- Blocking solutions
- Diluents
- Mounting reagents
- Enzymes
- Annex reagents
- Serums
Covid19
Documentation
ELISA technique
ELISA technique principle
ELISA is a technique commonly used in laboratories due to its low cost and ease to use.
At the start, this method was employed to replace the process of immunological bioassay using radioactivity.
ELISA enables the detection of an antigen or an antibody from a sample. An enzyme or a fluorochrome is linked to the antibody. When the antibody binds with the antigen, a colorimetric enzymatic or fluorometric reaction occurs producing a specific color. This color comes from the chemical transformation of the substrate by the enzyme.
ELISA can be either a quantitative or a qualitative method.
Qualitative: the bioassay indicates the antigen or the antibody’s presence in the sample.
Quantitative: the bioassay indicates the antigen or the antibody’s concentration in the sample. Thanks to the calibration curve, either optical density or fluorescence values give access to the concentration.
There are various methods available to perform an ELISA technique.
• There is the direct ELISA method: the primary antibody is linked to an enzyme/ fluorochrome.
• There is the indirect ELISA method: the secondary antibody (against rabbit or mouse, for example), is link to an enzyme/fluorochrome.
The indirect method is more widely used.
ELISA technique methods
There are four ELISA bioassay techniques: direct, indirect, sandwich and competitive.
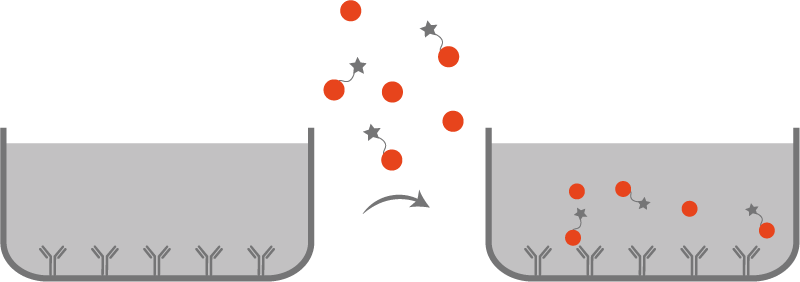
Direct ELISA
• Coating : antigen
The antigen is coated on the surface of the plate.
• Capture
The antibody is linked to a specific enzyme. This antibody is specific for the coated antigen.
• Detection
The substrate is added to enable the colorimetric reaction with the enzyme/fluorochrome.
Advantages:
• Simple and rapid: only primary antibodies are required. This process requires few steps.
• Mistakes are less likely to occur. There are fewer steps. Therefore, measurement mistakes are less likely to occur. There is no secondary antibody. Therefore, the is no cross-reaction risk.
Disadvantages:
• Low sensibility: there is no signal amplification.
• The development of this method takes time: for each target, a specific linked enzyme/ fluorochrome antibody must be tested which is expensive.
• The reactivity between the antibody and the antigen is limited: available space around the antibody is reduced by the enzyme/fluorochrome presence. Therefore, it may prevent it from binding to the antigen.
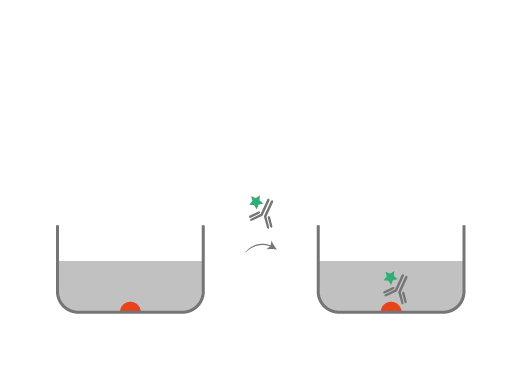
Indirect ELISA
• Coating: antigen
The antigen is coated on the surface of the plate.
• Capture
The primary antibody reacts and binds with the antigen.
• Amplification
An enzyme/ fluorochrome- linked secondary antibody is added. It will react against the primary antibody.
• Detection
The substrate is added and reacts with the enzyme/ fluorochrome and enables the detection of the antigen of interest.
Advantages:
• Flexibility: numerous secondary antibodies are available on the market.
• Sensibility: numerous secondary antibodies can link one primary antibody. Indeed, every primary antibody has several epitopes.
• A higher immunological reactivity: space around the primary antibody is enzyme/ fluorochrome free. The reactivity between the antibody and the antigen is not limited by steric effects.
• Economical: there is no need for coupled-enzyme/fluorochome primary antibody.
Disadvantages :
• A complex workflow: an additional incubation step is required for the secondary antibody.
• A possible cross-reactivity: the secondary antibody may react with the wrong antigen and induce a non-specific signal and some background sound.
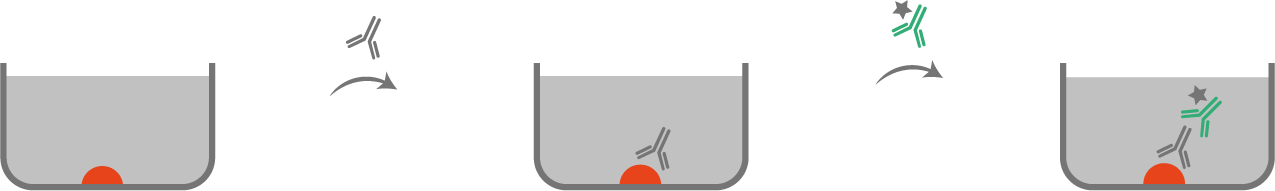
Sandwich ELISA
For this Technique, there are two antibodies. The antigen of interest will be trapped between those two antibodies.
• Coating: primary antibody
The primary antibody is coated on the the surface of the plate.
• Capture
The sample containing the antigen to be detected is added which will react with the coated antibody.
• Amplification
A second enzyme/ fluorochrome linked antibody is added (detection antibody). It will react with the antigen captured by the primary antibody.
Comment: both antibodies react on different epitopes.
• Detection
The substrate is added and reacts with the enzyme/ fluorochrome and enables the detection of the antigen of interest.
Advantages:
• Flexibility and sensibility: both indirect and direct method may be used.
• High specificity: two antibodies are used for the antigen detection.
• Compatible with complex samples: there is no need for an antigen purification.
Disadvantages:
• A complex workflow: there are additional incubation steps
• Needs to be optimized: cross-reactivity with these various antibodies must be check. Two antibodies against different epitopes from the same antigen are needed.
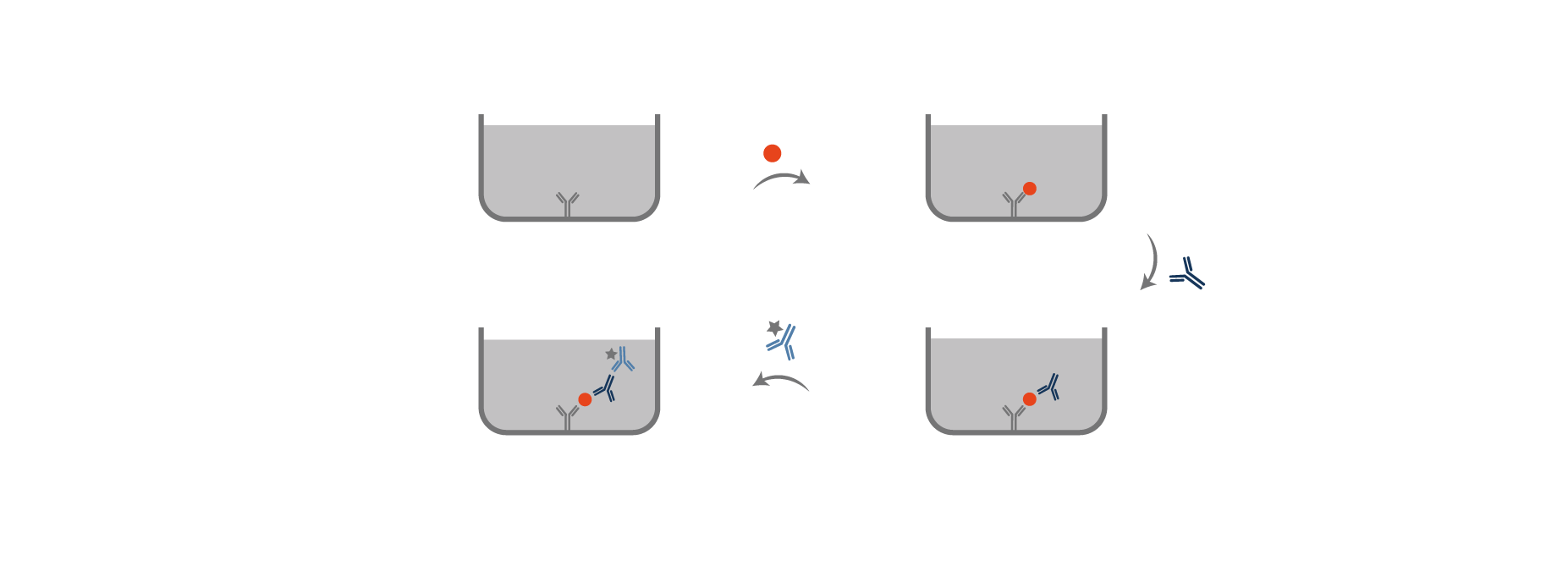
Competitive ELISA
• Coating
Antibodies against the antigen of interest are coated on the plate.
• Capture
Enzyme-linked antigen (at a precise concentration which is the reference) is added at the same time as the antigen sample to measure. These antigens compete with each other’s to bind to the pre-coated antibody on the plate.
The more the antigen of interest concentration is high in the sample, the least enzyme linked antigens will bind with precoated antibodies.
Competitive ELISA technique is optimal to detect small-sized antigens and with the use of one antibody.
Advantages :
• Largest flexibility: could be used with direct, indirect and sandwich ELISA methods.
• Higher specificity: the antigen of interest is specifically captured and detected.
• Robust technique: sample dilution impact is reduced
Disadvantages :
• Takes time: the protocol is complex
• Depending on the method applied, their disadvantages has to be taken into account.
ELISA reagents
To help you to enhance your results, please refer to the following products.
Blocking solution
Product name | Composition | References |
|---|
The Blocking Solution | This is a blocking buffer composed of casein molecules. These proteins bind to free sites on the plate and reduce non-specific binding | 110-050 110-125 110-500 |
SmartBlock | This is a free-BSA blocking buffer composed of synthetic peptides. These peptides can detect molecules such as nucleic ones. | 113-125 113-500 |
BSA-Block | This is a blocking buffer composed of BSA. | 115-125 115-500 |
Buffers
|
Stabiliser
Product name | When use it | References |
Antibody Stabilizer Tris (available with PBS) | This is a sample and antibody dilution buffer. It enables to keep it a longer time at 2-8 °C.
| 130-050 130-125 130-500 |
Liquide Plate Sealer | This buffer stabilizes antibodies and antigens once they are fixed on the plate. | 160-050 160-125 160-500 |
LowCross HRP-STAB | This is a dilution buffer for long term HRP linked antibody storage. It reduces non-specific binding cross-reactivity and matrix effects. | 270-050 270-125 270-500 |
HRP-Protector | This is a dilution buffer for long term storage of HRP linked antibodies. | 222-050 222-125 222-500 |
